Conduction and breakdown in liquids Liquid dielectrics are used as insulator in transformers, capacitors, high voltage cables, circuit breakers, …etc. Liquid dielectricsare normally mixtures of hydrocarbons and arweakly polarized.
They must be free from moisture, oxidation and water when used for electric insulation. Transformer oilis commonly used in power apparatus since it is cheap and colourless, mixture of hydrocarbon and paraffin. This can heat up to 95°but it becomes dark by time due to the formation of acids and resins. Silicon oilis also used for high temperature over 150°but is expensive. It has an inferior nonflammable properties. Organic estershave high boiling point and good temperature-viscosity relationship, and are used in capacitors. High temperaturehydrocarbon oil(HTH)or perfluoropolyether possess higher fire points, higher viscosity and lower heat transfer capability while tetrachloroethyleneC2Cl4has lower viscosity and higher heat transfer properties, see tables
Conduction and breakdown in Pure liquid
The electrodes used for breakdown voltage are spheres of 0.5 to 1 cm diameter with gap spacing of 100 to 200 µm. the voltage applied for breakdown test is low of order 50 to 100 kV and the breakdown strengths and conductivities in pure liquid are very high of order 1 MV/cm and 10-18to 10-20mho/cm When low electric fields < 1 kV/cm are applied, conductivities of 10-18-10-20are obtained. When the fields increase over 100 kV/cm the current is rapidly increase to saturated value, then generated because of the field aided electron emission. In a similar way of Townsend primary and secondary ionization in gases, the breakdown in liquid occurs at high fields when positive ions arrive to cathode to produce more electrons. Maximum breakdown strength are seen in the table. Also the figures show the breakdown with oxygen and hydrostatic pressure
Conduction and breakdown in Commercial liquid commercial liquid is not chemically pure and have gas bubbles, suspended particles …etc. The breakdown in commercial liquid depends on electrode condition and nature, physical properties of the liquid and impurities and gases present in the liquid. Suspended particle theory
A solid spherical particle of radius rand permittivity ε2experiences force F due to an applied electric field E, where ε1= permittivity of the liquid The breakdown strength of liquid containing solid impurities has lower value than of pure liquid
Bubble theory
A bubble of radius rand permittivity ε2in a liquid of permittivity ε1 and surface tension σ expresses a breakdown field Eodepends on initial size of bubble given as:
Vbis the voltage drop in the bubble
Stressed oil volume theory
The breakdown strength is determined by weakest region = largest impurity at maximum Emeasured by stressed oil volume CC. see figure
Vis the breakdown voltage, dis the gap length, kand nare constant, n < 1. the breakdown strength depends on the time and mode of applied voltage, it determined in the oil by exponential investigation. The breakdown in high purified liquid is given in the table:


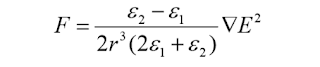


No comments:
Post a Comment